Executive Abstract
Since its introduction, the term “ecological novelty” has generated quite a lot of controversy in the scientific community. In response to repeated cycles of critiques and responses among proponents and detractors, the definition has been tweaked again and again. Yet the term remains more or less binary; a certain place either is or is not novel. Such a definition requires an enormous amount of elaboration on the specific terms – novel relative to what? measured how? – in order for each application of novelty to be understood in context. And comparing novelty among different places is nearly impossible. Due in part to these limitations, scientists are considering alternative ways to define novelty on a spectrum. Such a continuous, quantitative approach would address much of the recent controversy, and open up avenues for new scientific understanding.
Preamble
With increasing attention focused on anthropogenic alterations to global ecosystems (e.g., “novel,” “no-analog,” or “emerging ecosystems”), moving beyond categorical classifications of novelty is useful. The original conception of novelty was binary; either a place was or was not novel. Many scientists feel that some of the controversy that has shadowed the term novelty since its introduction may have been avoided if the original authors had proposed a “spectrum” of novelty. Such a continuous spectrum would avoid difficult questions with the current definition of novelty. For example, since human activity has chemically modified the Earth’s atmosphere, could one argue that the entire Earth is then novel? And, if the whole planet is novel, is there any utility in labeling particular places or habitats as such?
Some of the advantages conferred by quantifying novelty in a continuous, rather than binary, way include:
1) prioritization of management or restoration goals
2) better accommodation of a non-binary and very complex world
3) partitioning variance in communities among multiple axes of abiotic and biotic change
4) communication and public outreach
However, quantifying novelty in a robust and meaningful way can be challenging.
Quantifying Abiotic Novelty
In some ways, because of the wealth of both historical and projected future data, quantifying novelty of climate or other abiotic factors is more tractable than quantifying biotic novelty. Williams and colleagues (2007) present an example of this by taking four climate parameters (summer and winter temperature and precipitation) from the Intergovernmental Panel on Climate Change projections from the year 2100. The authors quantify abiotic novelty as the dissimilarity (measured as the standardized Euclidean distance) in future climate space relative to present day conditions (Fig. 1). Because temperature and precipitation are important determinants of many ecological processes, shifts in climate such as those mapped in Figure 1 will likely impact biotic communities as well. In addition to the above quantitative assessment, the authors provide a conceptual figure (Fig. 2) demonstrating likely extinctions and novel species assemblages that may result from these projected future climates.
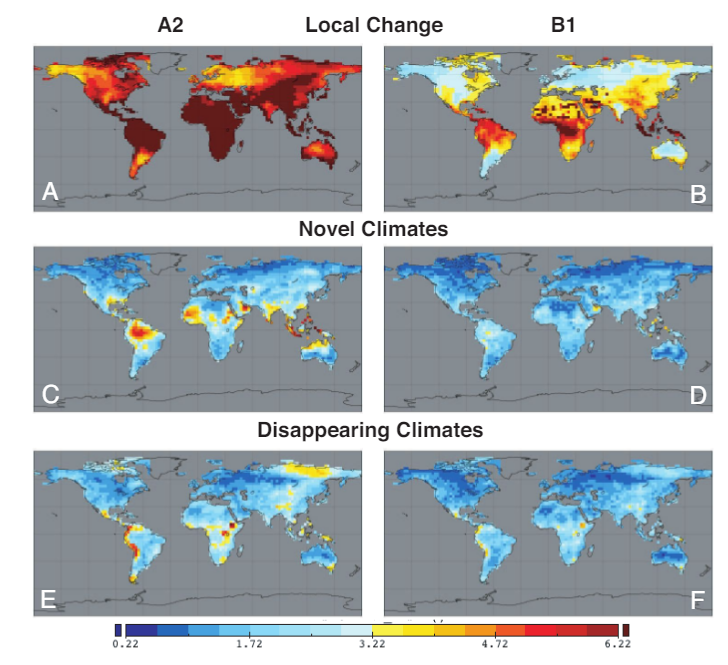
Figure 1 (from Williams et al. 2007, PNAS). Global maps of projected 2100 local climate change, arising novel climates, and disappearing climates based on the IPCC A1 (rapid; A, C, E) and B2 (mild, B, D, F) climate scenarios. Models were built using temperature and precipitation data from summer and winter months.
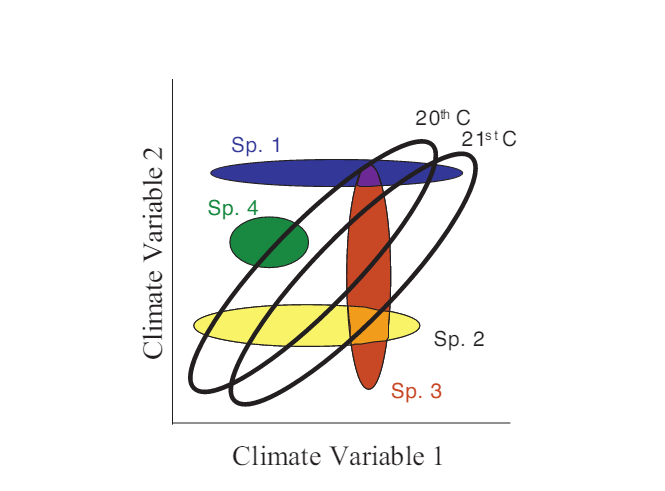
Figure 2 (from Williams et al. 2007, PNAS). Conceptual figure diagramming the fundamental niches of four species (colored ellipses) and potential future shifts under novel climate parameters.
Quantifying Biotic Novelty – Challenges
Quantifying biotic novelty presents an additional set of challenges and considerations, especially if the goal is to compare multiple measures of novelty across multiple communities. Historically, these comparisons have tended to be more descriptive than hypothesis driven (Weinstein et al. 2014), and are complicated by the fact that opposing responses to global changes at the species-level tend to render the corresponding community-level changes weak or undetectable (Supp and Ernest, 2014). Furthermore, comparisons of novelty (where novelty is quantified using dissimilarity in species composition as a proxy of novelty) across multiple study systems are also difficult because measures of beta diversity are sensitive to both sampling effort and regional species pool (Bennett and Gilbert 2015).
Another challenge for comparing biotic novelty among systems or regions is that many of the methods recently developed (e.g., Baselga 2010, 2012, Podani and Schmera 2011, Carvahlo et al 2012, Almeida-Neto et al 2013) employ multivariate methods originally designed for spatial comparisons among sites to describe temporal differences among communities. Temporal change has several unique properties (e.g., lack of physical boundaries, unidimensionality, autocorrelation, and directionality) that must be taken into account in analysis, making spatial measures of change a clumsy tool for time series data (Shimadzu et al 2015, Dornelas et al 2013).
Due to the challenges described in the above two paragraphs, comparisons of biotic novelty across space or time require carefully matching analytical methods to the question(s) and dataset(s) of interest. Relevant tools, methods, advice, considerations, etc. are described in the following paragraphs.
Quantifying Biotic Novelty – Some Best Practices
Much of the recent literature focused on quantifying differences between communities in space and time via alternative formulations of beta diversity is authored by Baselga (2010, 2012, 2013). His metric produces a multiple-site dissimilarity measure (can also be formulated to produce pair-wise dissimilarity, depending on data set) which separates beta diversity into two components: turnover (i.e., species replacement) and nestedness (i.e., species loss) (Fig. 3). Baselga argues that these two processes represent unique ecological phenomena, and disentangling their relative contributions can test interesting and relevant hypotheses that aren’t possible with traditional measures of beta diversity. This metric seems to have been widely adopted: the corresponding R package has been cited 62 times (as of 2016), while the 2010 paper that introduces his framework has been cited 267 times.

Figure 3 (from Baselga, 2010 Global Ecology and Biogeography). Conceptual figure demonstrating differences between turnover and nestedness. Both processes can contribute to biotic novelty.
Comparing changes in the novelty of the same community across time is best accomplished with measures of change that are explicitly formulated to describe temporal processes. Observations of temporal change can result from four different processes: measurement error, process error (i.e., significant drivers of change not considered in model), historical influence (i.e., autocorrelation—past community can influence contemporary community, but not vice versa), and systematic change (Dornelas et al. 2013). Shimadzu et al (2015) present a metric for comparing community change that accommodates these properties of temporal change. They break temporal turnover into two components – change in community composition and change in community size (aka capacity) – and from these components (with some fancy math) calculate a metric that, when tested with both real and simulated data, offers additional insights into temporal dynamics as compared with the borrowed spatial metrics. One such insight is the ability to identify any species AND any environmental factors which are important in driving the observed change.
A Very Simple Metric of Biotic Novelty
Perhaps the simplest metric for quantifying novelty is proposed by Helm et al (2015). They formulate a new index “Index of Favorable Conservation Status” which is calculated as the log ratio of characteristic:derived diversity, where characteristic diversity is that which should be there (i.e., native species in historical range) and derived diversity are those species which shouldn’t be there (i.e., non-native species or species not in historical range). Although this metric involves subjective decision making, there is value in this approach. It is simple and expert-based and therefore could be useful for managers and decision-makers, if not for ecologists. The authors suggest that this index captures conservation needs and adverse responses to global change in relative terms, and can be compared across regions and habitat types, can also be used to measure restoration success.
Closing Thoughts
There is plenty of room for development and advancement of analytical techniques that measure novelty. At current, we define ecological novelty much the same way Supreme Court Justice Potter Stewart described his threshold test for obscenity: “you know it when you see it.” Such qualitative assessments could be improved through the testing and development of rigorous quantitative techniques to describe and make inferences about novel ecosystems.
~by Amy Alstad~
Baselga, 2012. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecology and Biogeography.
Baselga 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography.
Bennett and Gilbert 2015. Contrasting beta diversity among regions: how do classical and multivariate approaches compare? Global Ecology and Biogeography.
Dornelas et al. 2013. Quantifying temporal change in biodiversity: challenges and opportunities. Proveedings of the Royal Society B.
Helm et al 2015, Characteristic and derived diversity: implementing the species pool concept to quantify conservation condition of habitats. Diversity and Distributions.
Shimadzu et al. 2015. Measuring temporal turnover in ecological communities. Methods in Ecology and Evolution.
Supp and Ernest. 2014. Species-level and community-level responses to disturbance. Ecology.
Weinstein et al. 2014. Taxonomic, phylogenetic, and trait beta diversity in South American Hummingbirds. The American Naturalist.
Williams, Jackson and Kutzbach. 2007. Projected distributions of novel and disappearing climates by 2100. Proceedings of the National Academy of Science.