What is Behavioral ecology and why should we care?
The natural world is organized into a nested hierarchy; genes are nested within individuals, individuals within populations, populations within communities, etc. As ecologists, we are often interested in understanding how biotic and abiotic components of the environment and their interactions affect the abundance and distribution of living organisms, and in turn, the flows of nutrients and energy through an ecosystem. Consequently, higher levels of organization (populations and communities) have traditionally captivated much of the attention by ecologists, where population- and community-ecology typically treat individuals as being more-or-less ecologically equivalent. However, variation in traits, such as behavior, morphology, physiology, among individuals of the same species, sometimes even within the same population can exceed the variation observed among species by as much as 200%! (Ref). Moreover, because natural selection occurs at the individual level, studying the fitness consequences of individual variation can help bridge the divide between ecological and evolutionary processes (Bolnick et al. 2011). This is precisely the aim of behavioral ecology– to study how natural selection shapes behavior. As many of the ecological processes are explicitly behavioral, including habitat selection, predator-prey interactions, inter- and intra-specific competition, knowledge about how natural selection shapes behavior is critical to understand how the environment shapes, and is shaped by, behavioral interactions among individuals. This knowledge is fundamental to our understanding and ability to mitigate the potential impacts resulting from human-induced rapid ecological changes (HIREC; Robertson et al. 2013; Macdonald 2016).
Ecological novelty and the yin and yang of behavior
are defined as anthropogenically driven changes (e.g. habitat loss, climate change, the spread of exotic/invasive species including diseases) to an ecosystem or biosphere that occur at a historically rapid pace (Sih et al. 2011). Because behavior is the fastest, most flexible, and reversible way for an individual to respond to environmental change, behavior can act to moderate, or amplify the effects of ecological novelty, depending on the predictability of environmental variation (Myers and Bull 2002) and the preference for a behavioral option to increase relative to its fitness value (Roberston et a. 2013). The most apparent way in which behavior can amplify the effects of ecological novelty is through behaviorally mediated trophic cascades (Schmitz et al. 1997) (Fig. 1). For example, when large carnivores are removed from a system, smaller meso-predators are ‘released’ from the fear of being eaten and shift their foraging behaviors, ultimately to the peril of organisms occupying lower trophic levels such as primary producers (vegetation) and consumers (herbivores) (Schmitz et al 1997, Estes et al. XXX). In this example, the ability of meso-predators to perceive and respond to altered cues in the environment by switching their foraging behavior is a form of behavioral flexibility. If this flexibility in foraging behavior confers some kind of fitness advantage to meso-predators, then this example shows how behavior can both amplify (by way of trophic cascade) and moderate (through population enhancement) the effects of ecological novelty, which was in our case the loss of a large carnivores. Alternatively, if behavioral flexibility caused a reduction in fitness, as would be the case if the cues used by meso-predators to sense danger had been altered or removed but the threat still remained, then this maladaptive behavioral flexibility could facilitate ecological novelty through the emergence of an ‘evolutionary trap’ (Sih 2004, Robertson et al. 2013).
Figure 1. Example of a novel ecosystem created from a behaviorally mediated trophic cascade. In the absence of cues from a large predator (gray silhouetted wolf), mesopredator abundance and foraging activity increased (black silhouetted raccoon), leading to a reduction in crabs (gray crab silhouette) which resulted in an increase in sculpin (crab competitor) and snails (crab prey). When predator cues were present (black silhouetted wolf), raccoons decreased foraging activities resulting in an increase in crab populations and a subsequent decrease in snails and sculpin. Figure adapted from Suraci et al. 2016 and inspired by Hobbs et al. 2009.
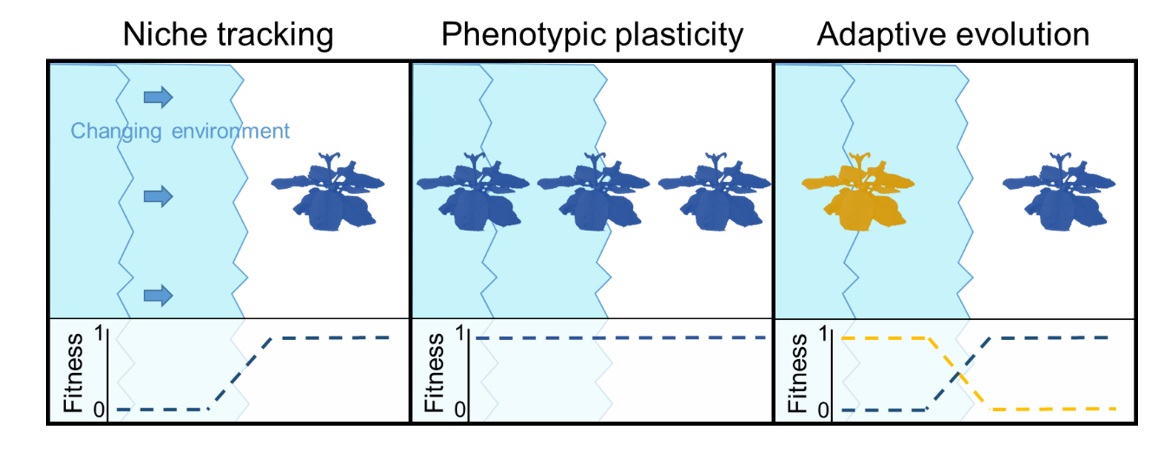
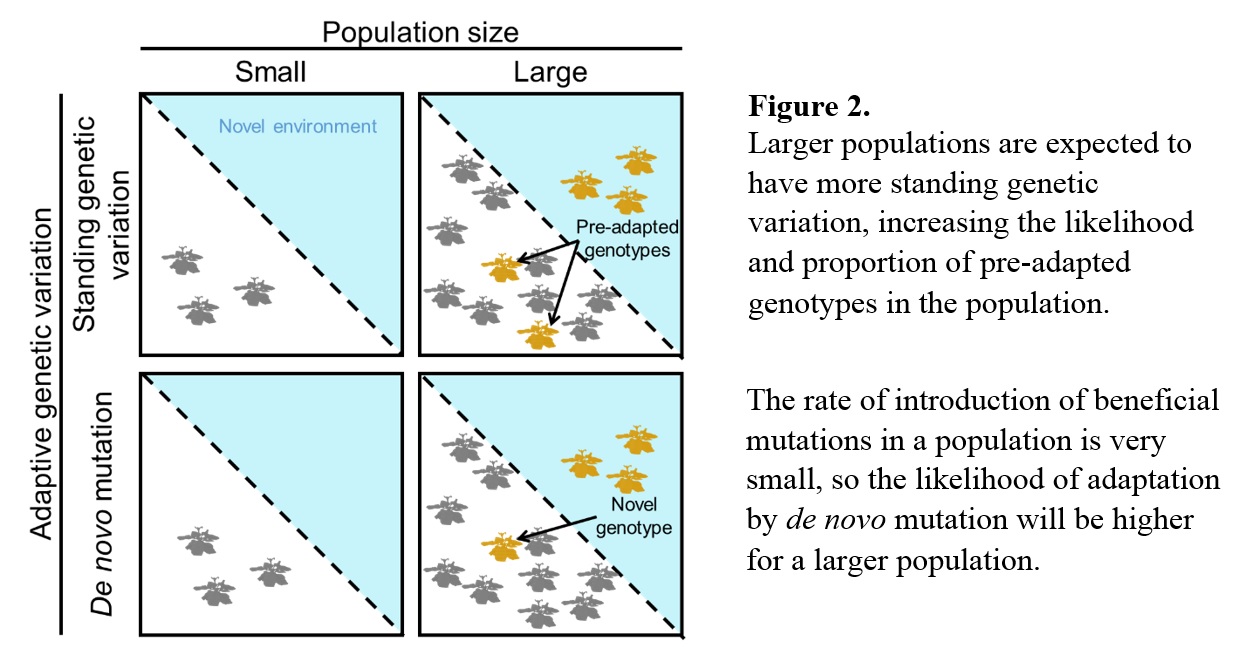
Ultimately, novel ecosystems exist along a continuum of human-induced rapid ecological change. As highlighted above, the ability of individuals to express different phenotypes (behavioral, physiological or morphological) across a range of environmental conditions – a phenomenon known as phenotypic flexibility – could prove advantageous or maladaptive in novel ecosystems depending on the accuracy of information (cues) that are available for individuals to predict future environmental conditions. Therefore, gradients of novelty can be used as natural ‘titration’ experiments to test theories about adaptive phenotypic plasticity. Such tests could provide valuable insight on the causes and consequences of phenotypic plasticity and generate ‘out-of-the-box’ solutions to conservation problems. A few examples of such creative solutions include: breeding programs that select for plastic genotypes, or training animals (https://www.youtube.com/watch?v=gSCr5OxXN1A) to perform novel behavioral tasks that could help them persist in the face of environmental challenges.
~by Chris Latimer~